Once the molecule file is fully loaded, the image at right will become live. At that time the "activate 3-D" icon ![]() will disappear.
will disappear.
Serum Albumin
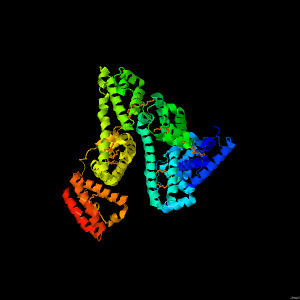
Human serum albumin is an abundant blood protein. Its role is to carry fatty acids in the circulation, since fatty acids are otherwise insoluble in an aqueous environment. It exists as an alpha helical monomeric protein. Each protein can carry seven fatty acid molecules.
Like most globular proteins, its three dimensional fold is formed with a hydrophobic core.
Acidic and basic and other less hydrophobic amino acid sidechains face the outside of the protein. These sidechains are able form hydrogen bonds with surrounding water molecules allowing the protein to be soluble.
Slicing our way down through the protein, we can see the prevalence of hydrophobic residues towards the centre.
Seven fatty acids located in hydrophobic pockets of this serum albumin structure. Watch as we slice back to the top.
The structure used for this presentation is from PDB entry 1GNJ reported by Petitpas et al., 2001 J. Mol. Biol. 314, 955-960.
(for more information about human serum albumin see this site at the PDB).
Prepared by Jackie Wilce, Monash University
Page skeleton and JavaScript generated by the Export to Web module of Jmol 14.29.29 on 04/01/2019.
Page skeleton and JavaScript generated by the Export to Web module of Jmol 14.29.29 on 04/01/2019.