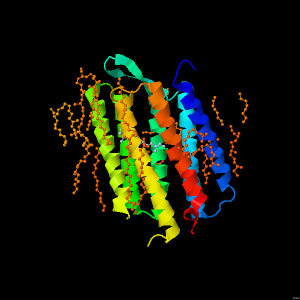
Bacteriorhodopsin - An alpha helical membrane spanning protein
Bacteriorhodopsin is a membrane spanning protein from a halophilic (salt loving) bacteria that functions as a light-driven ion pump. It forms a trimer, with each monomer forming a seven transmembrane alpha helical bundle. In the centre of each alpha helical bundle is a retinal molecule that undergoes isomerisation in response to light. This triggers a protein conformational change that results in the transfer of a proton across the membrane. Shown is one of the alpha helical bundles.
The retinal molecule is covalently linked to Lys216.
This protein structure is not formed with a hydrophobic core. Rather, hydrophobic amino acid residues form a hydrophobic outer surface that can interact with the lipid bilayer.
In this particular crystal structure, lipid groups were co-crystallised with the protein, and thus can also be seen.
Charged amino acid side chains Lys, Arg, Glu and Asp are also prevalent at the top and bottom of the protein that are exposed to the aqueous environment.
The helices are held together via hydrogen bonds between main chain and sidechain groups. Illustrated here are just four examples of hydrogen bonds that occur in the interior of this protein to hold the helices together.
In this crystal structure some water molecules can also be observed.
Note that these tend to be at the aqueous exposed ends of the molecule or within the molecule, rather than at its hydrophobic surface that is embedded in the membrane.
The structure used for this presentation is from PDB entry 1C3W reported by Luecke et al., 1999 J. Mol. Biol. 291, 899-911.
(for more information about bacteriorhodopsin see this site at the PDB).
Prepared by Jackie Wilce, Monash University
Page skeleton and JavaScript generated by the Export to Web module of Jmol 14.29.29 on 04/01/2019.