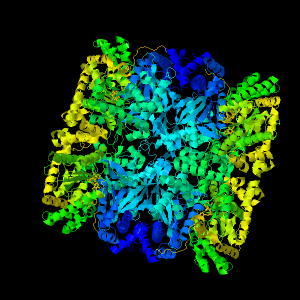
Glycogen Phosphorylase
Glycogen phosphorylase is an enzyme that catalyzes the phosphorolytic cleavage of glycogen between its alpha-1,4-linked glucose units, thereby producing alpha-D-glucose-1-phosphate. Click here for a tour of the glycogen phosphorylase enzyme.
This enzyme has crystallised as a tetramer (with each monomer in its active form, or "R" form). However, it usually acts as a dimer.
Each subunit consists of about 38 alpha helices
and seven beta sheets.
The catalytic binding site is located in the center of the enzyme. Numerous residues as well as pyridoxal phosphate (PLP), an essential cofactor, form the catalytic binding site.
Substrates enter via a channel that is partially blocked by a loop that consists of residues 282-285 (not shown). Shown here is the transition state analog, nojirimycin tetrazole (NJT), a competitive inhibitor.
A phosphate ion binds between the tetrazole ring of NJT and the 5'-phosphate of PLP.
Several changes to the protein conformation occur upon substrate or inhibitor binding in the catalytic site. When NJT binds, Leu 136 shifts to accommodate the tetrazole ring. His 377 also shifts. This residue may play a role in stabilizing the transition state.
Another shift involves the loop that blocks the catalytic site. When this loop shifts, it replaces the acidic Asp 284 residue (not shown) that was previously located in the catalytic site, with the crucial basic residue Arg 569. These conformational changes create a "high affinity phosphate substrate recognition site".
It also creates a favorable electrostatic pocket for the PLP 5'-phosphate group. This electrostatic environment is created by Lys 568, Arg 569, and Lys 574.
A number of aromatic compounds such as caffeine can bind near the entrance to the catalytic channel and act as activation inhibitors. The inhibitor binds between the aromatic rings of Tyr613 and Phe285 (not visible in this crystal structure).
(for more information about glycogen phosphorylase see this site at the Protein Data Base).
Prepared by Jackie Wilce, Monash University
Adapted from Charles Grisham's "Interactive Biochemistry"
Page skeleton and JavaScript generated by the Export to Web module of Jmol 14.29.29 on 13/01/2019.