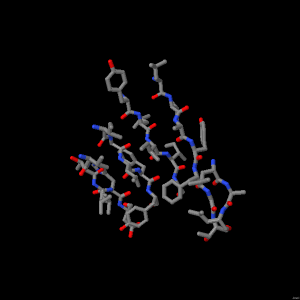
Protein Secondary Structure - Beta Sheet
The beta sheet is higher level of protein secondary structure first proposed in 1951 by Linus Pauling and Robert Corey.
(see http://www.pnas.org/site/misc/classics1.shtml).
The amino acid chains are in extended conformation running either parallel or antiparallel to one another. This beta sheet is made up of 5 strands. Spin the model to observe their arrangement.
The amino acid sidechains face outwards on both sides of the beta sheet. The spacefill model shows the volume that the sidechains fill and the ball and stick model is useful for examining the arrangement of the atoms.
The beta sheet structure is stabilised by hydrogen bonds formed between the chains between backbone C=O and H-N groups. Click the button below to display the hydrogen atoms and the hydrogen bonds in this beta sheet.
The beta sheet is sometimes depicted as a set of ribbons, with an arrow showing the N- to C-terminal direction of the amino acid chains. This cartoon depiction shows the curvature that the sheet can adopt.
There are five chains in this beta sheet. Each chain in the sheet is called a beta strand.
Page skeleton and JavaScript generated by the Export to Web module of Jmol 14.29.29 on 23/01/2019.