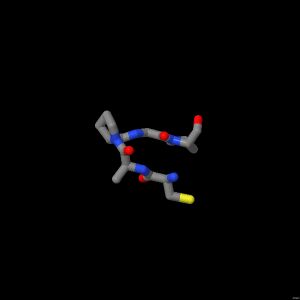
Beta turns
Beta turns, also known as beta bends or tight turns, are a type of secondary structure.
In a beta turn, a tight loop is formed which places residue (i) right alongside residue (i+3). i.e. a residue 3 down the chain.
In some cases the carbonyl oxygen of residue (i) forms a hydrogen bond with the amide proton of residue (i+3). In this example, however, the hydrogen bond is not formed.
Proline and Glycine are frequently found in beta turns, proline because its cyclic structure is ideally suited for the beta turn, and glycine because, with the smallest side chain of all the amino acids, it is the most sterically flexible.
A beta turn is a means by which the protein can reverse the direction of its peptide chain.
Beta turns often promote the formation of antiparallel beta sheets.
Streptomyces subtilisin inhibitor (shown here) uses a beta turn to connect two of its antiparallel strands.
Adapted from Charles Grisham's "Interactive Biochemistry"
Page skeleton and JavaScript generated by the Export to Web module of Jmol 14.29.29 on 23/01/2019.